Reducing medical plastic waste
Identifying and reducing specific fractions of operation room medical waste to enhance material circularity
Written by Tiffany Marilou Ramos, Roskilde Environmental Plastic Society (REPS), Roskilde University, tmramos@ruc.dk
Plastic products and packaging
Production of plastic products and packaging has continued to rise exponentially and become ubiquitous in all sectors, including healthcare. In addition to disease prevention, healthcare plastics are favored for low production costs, durability, and convenience.
Single-use plastic (especially in healthcare) follows a linear value chain where products are disposed after a single use. As a result, the global healthcare sector is responsible for 4.4 percent of global net carbon emissions due to resource intensive practices, the use of anesthetic gases, and material consumption (Karliner et al., 2019 ). Operating theaters require additional sterilization procedures and consumables to ensure patient and staff safety and therefore can be three to six times more consumptive compared to other departments within a hospital (MacNeill et al., 2017).
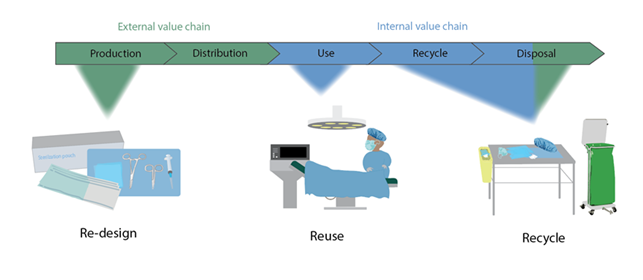
Efforts to curb plastic pollution often focus on waste management solutions or solutions at the end-of-life of products (Johansen et al., 2022), yet EU directives such as the Waste Framework Directive highlight that reduction efforts are better suited further up in the value chain before a product becomes waste (Waste Framework Directive, n.d.). As regulation in the European Union (EU) shifts towards reducing carbon emissions and increasing circularity of materials, the healthcare sector has a responsibility to reduce its resource consumption while providing the same level of quality care. While eliminating all healthcare plastics is impossible, focusing on “hotspot” products and procedures can have a major impact.
The aim of this collaboration between Region Sjælland in Denmark and Roskilde University is to highlight the composition of medical plastic waste in two hospitals in Denmark, observe processes of staff that would allow for reuse or sorting implementations in efforts to reduce medical single-use plastic products and packaging.
A mixed-method approach
This project adapted a mixed-method approach to gather qualitative and quantitative data from the hospitals and this was subdivided into three main categories:
1) Direct observations
Direct observations of surgical products and processes were conducted to assess the quality and quantity of single-use plastic fractions. Furthermore, polymer types of the products were identified, process flows of the staff were considered, and surveys involving the key staff members were conducted to include their expert opinions.
During this phase of the study, products were identified and categorized, and potential sorting implementations were considered. In total, 46 operations were observed within two separate hospitals in Region Zealand.
2) End-of-life effects of plastic-associated chemicals of medical products
After product types and composition were identified during the observations, the effects of plastic-associated chemicals on end-of-life options were addressed.
A literature review was conducted that reinstated the well-documented effects of chemical groups (Bisphenol A (BPA), Per- and polyfluoroalkyl substances (PFAS), and Phthalates which are all recognized by the EU as chemicals of very high concern to human health and the environment. Additionally, we assessed current EU legislations that include these chemical groups and concluded that comprehensive legislations on the mixtures of chemicals are lacking. Our review highlighted the novel hinderance of these chemicals on the circularity and recycling of materials containing them.
3) Life Cycle Assessment (LCA) between two types of sterilization packaging
Two products were chosen for comparison in a life cycle assessment. Both products are used for steam sterilization of surgical tools. One product is a nonwoven polypropylene sheet (also referred to as blue wrap) and the other is a rigid sterilization case (RSC) often made of aluminum and stainless steel. Processes surrounding these products revealed them to be relatively “easy” fractions of sortable high-volume waste.
Both blue sheets and RSCs are unpacked before the patient enters the room and either discarded or stored for reuse. In addition to the autoclave process, the RSC must undergo a disinfection process before reuse, requires more storage space, and is heavier in weight for the medical staff handling them. An LCA was conducted to assess the impacts (in terms of CO2 emissions) of both products from the extraction of raw materials to the current waste management processes of incineration or recycling.
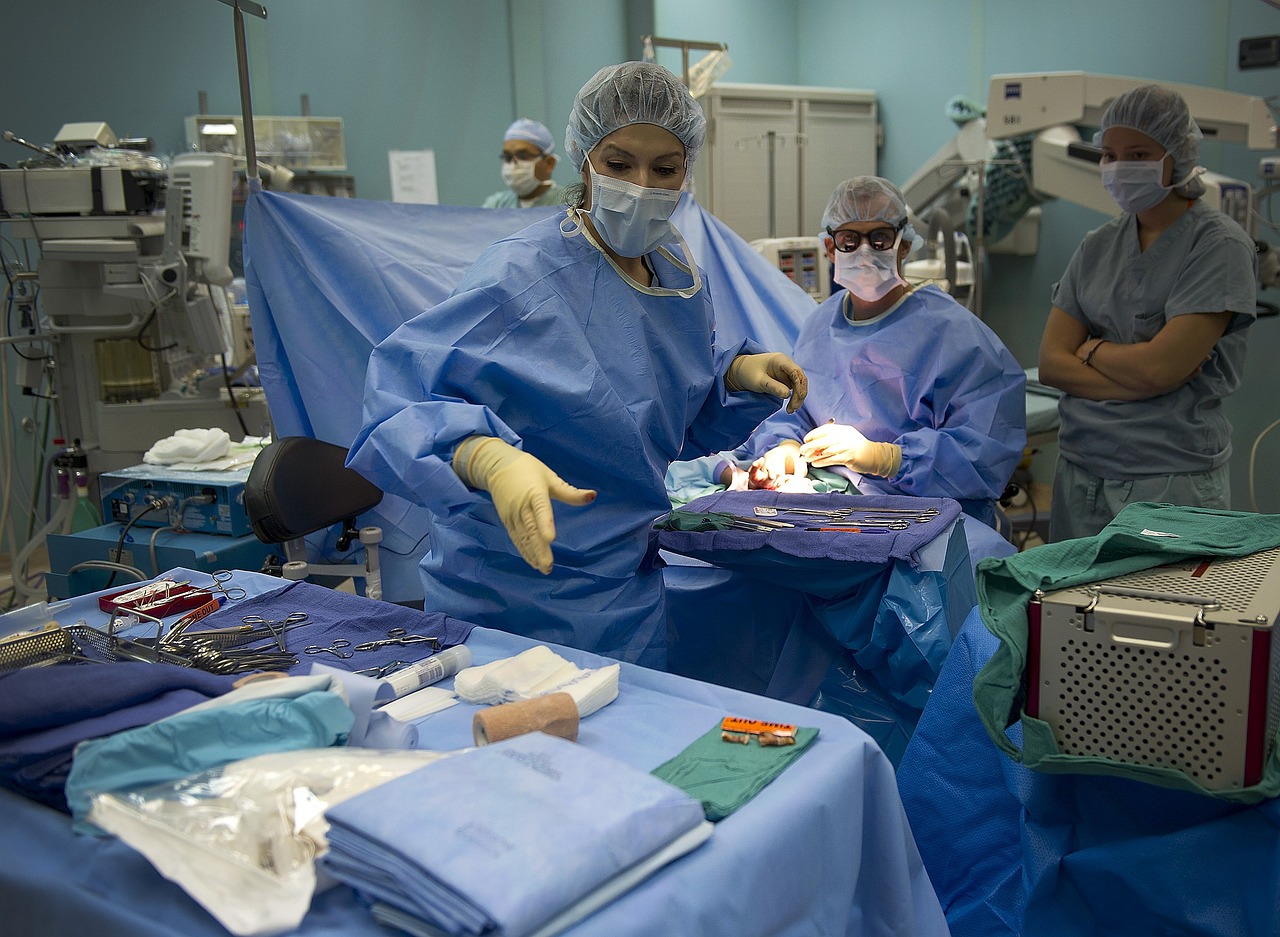
Credits: Tiffany Marilou Ramos & Nikoline Garner Oturai
Study results
We were able to categorize and compare single-use plastic waste among two different hospital types (specialized and general) and across different surgical departments (Ramos et al., 2023). Results from these observations provided insight into site-specific implementations of potential reuse and reduction efforts. Furthermore, current product design of medical products was highlighted as a major hinderance to recycling in terms of both on-site separation as well as chemical composition (Ramos et al., 2023).
Results from the life cycle assessment are still on going, but currently suggest that energy consumption from the sterilization and disinfection processes is the most resource intensive process throughout both products life cycles. We also observed a threshold of uses for the reusable sterilization cases to supersede the blue wrap.
From this study we were able to assess reduction efforts for single-use medial plastics from the production (design), use (reuse of sterilization packaging), and disposal phases (potential sorting implementations). Our study aims to provide hospitals, staff, producers of medical products, and legislators with to reduce plastic waste generation from the medical sector. Some of the recommendations mentioned call on producers to implement product designs that account for the waste management of a product and legislators both in the EU and globally to consider comprehensive legislation for reducing chemical mixtures of plastic products.
Edited by: Diya Chakravorty and Sara Plassnig
References
Johansen, M. R., Christensen, T. B., Ramos, T. M., & Syberg, K. (2022). A review of the plastic value chain from a circular economy perspective. Journal of Environmental Management, 302, 113975. https://doi.org/10.1016/j.jenvman.2021.113975
Karliner, J., Slotterback, S., Boyd, R., Ashby, B., Steele, K., Health Care Without Harm, & Arup. (2019). HEALTH CARE’S CLIMATE FOOTPRINT: HOW THE HEALTH SECTOR CONTRIBUTES TO THE GLOBAL CLIMATE CRISIS AND OPPORTUNITIES FOR ACTION. In https://noharm-global.org/. https://noharm-global.org/sites/default/files/documents-files/5961/HealthCaresClimateFootprint_092319.pdf
MacNeill, A., Lillywhite, R., & Brown, C. J. (2017). The impact of surgery on global climate: a carbon footprinting study of operating theatres in three health systems. The Lancet Planetary Health, 1(9), e381–e388. https://doi.org/10.1016/s2542-5196(17)30162-6
Ramos, T. M., Christensen, T. B., Bour, A., Almroth, B. C., Kristensen, D. M., Selck, H., & Syberg, K. (2023). A not so circular healthcare economy: A review of challenges with plastic associated chemicals. TrAC Trends in Analytical Chemistry, 166, 117191. https://doi.org/10.1016/j.trac.2023.117191
Ramos, T. M., Christensen, T. B., Oturai, N. B., & Syberg, K. (2023). Reducing plastic in the operating theatre: Towards a more circular economy for medical products and packaging. Journal of Cleaner Production, 383, 135379. https://doi.org/10.1016/j.jclepro.2022.135379
Waste Framework Directive. (n.d.). Environment. https://environment.ec.europa.eu/topics/waste-and-recycling/waste-framework-directive_en